MRGPRD (MAS related GPCR family member D) is a class A G protein coupled receptor with established roles in pain, itch, and inflammatory responses. It is an understudied receptor which represents an attractive yet challenging target for therapeutic development. Expanding the pharmacological toolkit available for studying MRGPRD is therefore essential to accelerate the discovery of novel agonists and antagonists.
To address this unmet need, BioAscent scientists collaborated with colleagues at the Medicines Discovery Catapult to develop and characterise a novel, label-free cellular impedance assay for MRGPRD. Cellular impedance is a dynamic, real time technique which is an excellent tool for characterising the molecular pharmacology and pathway coupling of GPCR targets, offering a powerful alternative to traditional label-based assays that measure downstream signalling.
Known MRGPRD agonist β-alanine1 was used to characterise receptor activity in the xCELLigence label-free assay. A multi-phasic response was observed, with a transient peak after 1 minute followed by a second sustained response (Figure 1).
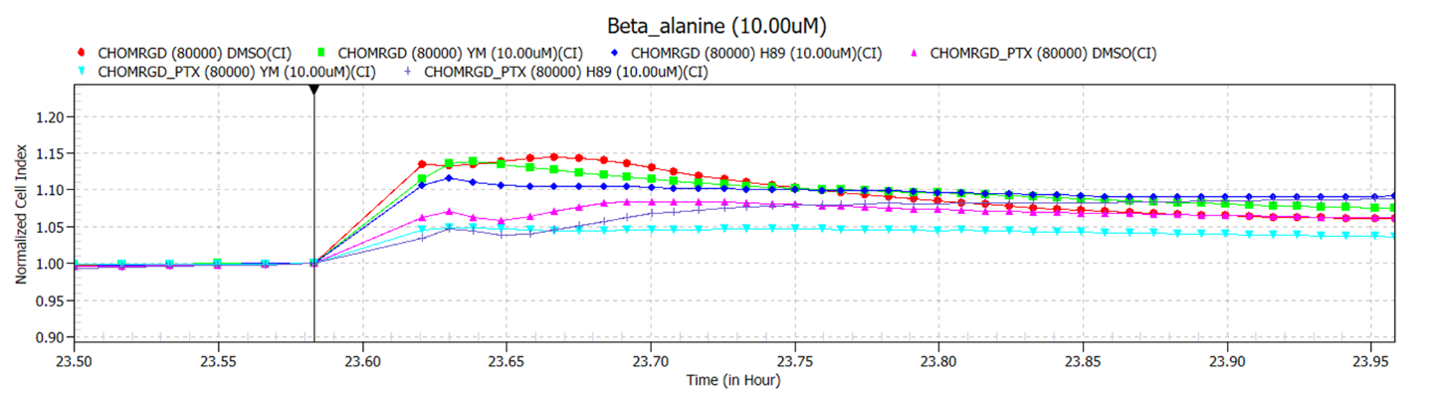
Figure 1: The β-alanine impedance response is a complex of various G protein signalling pathways: real-time xCELLigence trace of 10mM Beta-Alanine ± pertussis toxin (PTX) and/or YM-254890 and/or H89.
MRGPRD has been reported to couple to both Gαi/o and Gαq proteins.1-4 To study the contribution of each pathway we used YM-254890 to inhibit coupling via Gαq, and pertussis toxin (PTX) to inhibit coupling via Gαi (figure 1). We analysed the two peaks separately and found that the first peak appears predominantly related to Gi coupling, as it is significantly inhibited by PTX. Co-administration of YM and PTX almost completely abolished both responses, indicating that the second sustained response may be more closely related to the GPCR coupling through Gq. When the Gi response was inhibited using PTX, the second peak increased in size, suggesting that when the receptor can no longer couple through Gi it increases coupling via Gq to compensate.
To evaluate the suitability of cellular impedance as an orthogonal technology for MRGPRD ligand discovery, we compared these findings with data generated using a conventional intracellular Ca²⁺ mobilisation assay.
Using a CHO cell line stably expressing MRGPRD we optimised an intracellular Ca2+ release FLIPR assay. Concentration-dependent increases in intracellular Ca2+ were observed upon the addition of β-alanine. Inhibition of Gαq-G protein coupling using YM-254890 abolished Ca2+responses, demonstrating that the β-alanine response observed in the FLIPR is due to coupling via Gαq as expected.
We next used PTX to inhibit coupling via Gai resulting in an approximate 50% reduction in the β-alanine response indicating possible cross-talk causing a Gαq response to be inhibited when Gαi is inhibited. This could be because basal Gαi activity normally suppresses cAMP and keeps PKA low which supports IP₃/Ca²⁺ signalling; when this is inhibited via PTX, cAMP/PKA increases which could lead to PKA phosphorylating and inhibiting several components of the Ca²⁺ signalling pathway, resulting in a reduced Ca2+ FLIPR signal. An alternative possibility is that inhibiting Gαi with PTX could alter the receptor conformation, biasing the receptor toward conformations that couple less efficiently to Gαq.
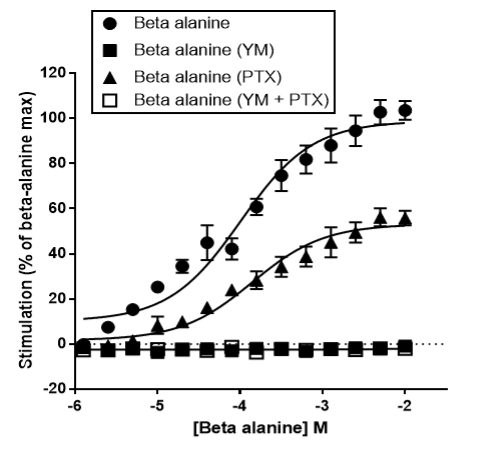
Figure 2: The effects of YM-254890, PTX and PTX plus YM-254890 on FLIPR Ca2+ assays were determined. Concentration-effect data are expressed as a percentage of the maximum response to agonist and are the mean ± SEM of a minimum of 5 replicates.
Our data highlights the complexity of GPCR pharmacology and the importance of employing orthogonal techniques to fully characterise receptor behaviour, inform decision making and derisk early-stage drug discovery.
We established a novel, label free cellular impedance assay capable of determining and confirming the pharmacological properties of molecules identified in more conventional second messenger screens. Studying the whole cell response to compound treatment reveals previously uncharacterised signalling dynamics, resulting in deeper mechanistic insight to guide medicinal chemistry and derisk early stage screening for this complex target.
Our bespoke and robust assay workflow for MRGPRD drug discovery enables the interrogation of receptor signalling and facilitates the identification of high quality tool compounds and potential therapeutic candidates, supporting the development of novel, targeted medicines for conditions including pain, itch, and inflammatory responses.
Learn more about BioAscent’s assay development and screening expertise here.
Shinohara et al. (2004) Journal of Biological Chemistry https://doi.org/10.1074/jbc.M314240200
Crozier et al. (2007) Journal of Neuroscience https://doi.org/10.1523/JNEUROSCI.4932-06.2007
Ajit et al. (2010) Journal of Biomedicine and Biotechnology https://doi.org/10.1155/2010/326020
Uno et al. (2012) Journal of Biomedicine and Biotechnology https://doi.org/10.1155/2012/816159